TGF-β Biology & Role in Diseases
At Keros, we are leveraging our protein engineering and drug development expertise to bring new treatment options to patients with rare diseases.
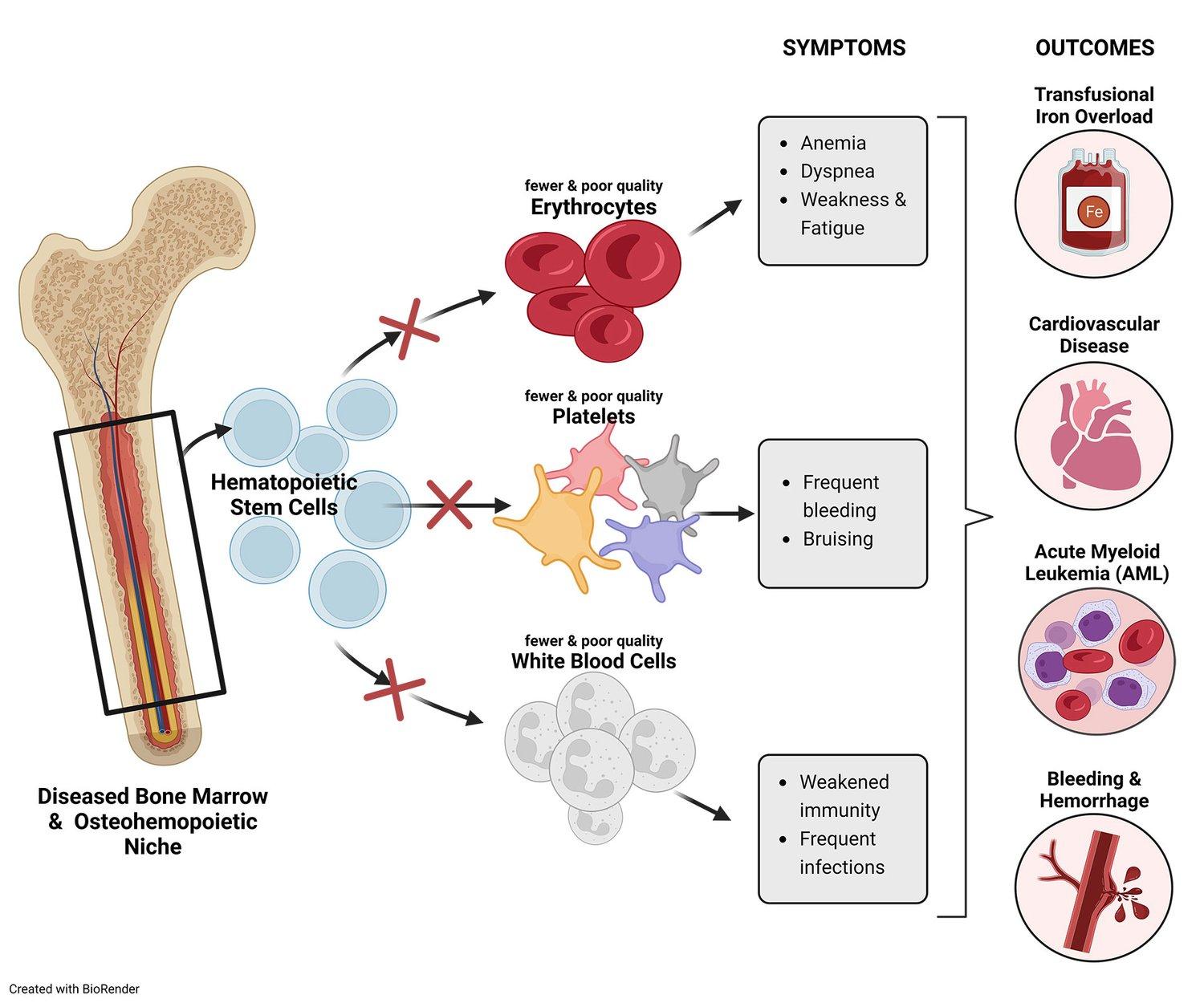
Our science focuses on the transforming growth factor-beta (TGF-β) superfamily—a broad and complex area of biology—comprised of more than 30 ligands, 12 receptors, numerous co-receptors and secreted regulatory molecules that play critical roles in tissue development and homeostasis. Accordingly, imbalanced signaling in the TGF-β pathway may cause or contribute to the progression and symptoms of these diseases. Through many years of research and clinical development experience, we have honed a deep understanding of TGF-β and have developed a pipeline of candidates aimed at treating diseases that are linked to imbalanced signaling of the TGF-β pathway.